The Scientific Industry Leader in USP<1062> Tablet Characterization and Methodologies
Are you seeking a trusted partner for USP <1062> tablet characterization and formulation development? Look no further than Natoli Scientific, a division of Natoli Engineering Company Inc. With our cutting-edge technology, extensive expertise, and commitment to excellence, we are at the forefront of USP <1062> Tablet Characterization, ensuring your tablet manufacturing process is optimized and compliant.
Why Companies Choose Natoli Scientific for USP <1062>?

Unmatched Expertise:
With our team of experienced scientists and engineers, we possess an in-depth understanding of USP <1062> and its implications for tablet formulation. We stay up to date with the latest industry advancements, ensuring you receive the most accurate and relevant guidance.

Advanced Equipment:
Our state-of-the-art R&D tablet presses, including the multi-station rotary tablet press, provide a comprehensive platform for collecting compaction data. We go beyond the basics, delving into the significance of independent variables and their impact on tablet quality during scale-up.

Comprehensive Insights:
Compressibility, compactibility, and tabletability are essential aspects of tablet formulation. Our expertise in these areas allows us to guide you in achieving optimal solid fractions, minimizing tablet porosity, and enhancing tablet mechanical strength. By adhering to USP <1062> standards, you can ensure consistent and reliable tablets.
DON'T BE DECEIVED BY YOUR DATA:
In the intricate world of tablet compression, shaped tooling consistently stands out, primarily due to the comprehensive, incisive data it discloses about your tablet formulation during the compression process. Its aptitude for constructing complex shapes and applying diverse forces provides a matchless insight into the distinctive properties and responses of your formulation throughout compression. This is particularly crucial when flat-face punches might conceal issues such as capping, low hardness or strength, friability, and dissolution problems. Understanding that if the final tablet shape varies from what is tested, the collected data becomes solely applicable to those unique conditions is fundamental. Simply put, it’s the science of the process.
As your operations expand to cater to heightened production volumes, the data harvested via shaped tooling becomes indispensable, offering a reliable blueprint for your formulation’s behavior under varied compression scenarios. Unlike the confined outlook provided by flat-face tooling, shaped tooling bestows a comprehensive understanding of your formulation’s performance across different designs. Moreover, it enables superior control over compaction time and force distribution, pivotal factors in ensuring tablet quality and consistency. Why constrain your growth with substandard data?
Avoid the pitfalls of flat-faced testing that could potentially hamper your tablet manufacturing process.
Instead, choose shaped tooling testing and unveil a plethora of data that can adeptly steer your production process. The core message is clear: Cup Configuration significantly influences USP<1062> accuracy. Break away from the limitations of flat-faced testing. Let Natoli Scientific be the catalyst in your tablet compression transformation.
Natoli Scientific USP <1062> Timeline
Select a year below to see the Natoli history with the the USP <1062>.
First Time Tablet
Profiles Published
Joris et al. published an article showing Compression Behavior of Orthorhombic Paracetamol
First Natoli Tablet Press & Tooling Applied Learning Experience Class

Dr. Haware from the Natoli team started using the original compressibility, compactibility, and tabletability profiles
The United States Pharmacopeia (USP) publishes a draft of USP <1062> for public comment.

- Natoli Scientific team member Robert Sedlock published articles on tabletability, compactibility, and compressibility.
- Natoli Scientific conducted the Tablet Press & Tooling Applied Learning Experience Class.

- The USP finalizes and publishes USP <1062> in the USP 40th Edition.

- Natoli Scientific conducted the Dry Granulation Formulation & Tableting Techniques - Webinar.
- Natoli Scientific published a blog post on closing the "knowing-doing" gap in R&D using USP <1062> data.
- The International Pharmaceutical Federation (FIP) adopts USP <1062> as a global guideline for the characterization of solid oral dosage forms.
- USP <1062>
Chapter, including compressibility, compactibility, and tabletability profiling, is officially published. - The European Directorate for the Quality of Medicines & Healthcare (EDQM) adopts USP <1062> as a guideline for the characterization of solid oral dosage forms.
- Natoli Scientific had already been practicing "USP<1062>" before its official inclusion in the USP

- Natoli Scientific conducted the Multi-Tip Tooling, Proper Tooling Setup & Installation Class.
- Natoli Scientific conducted the Tablet Press & Tooling Applied Learning Experience Class.
- Natoli Scientific conducted the Wet Granulation Formulation & Tableting Techniques - Webinar.
- Natoli Scientific conducted the Tablet Characterization with Regulatory Compliance Methods – USP<1062> class.
- Natoli Scientific participated in the Virtual Pharma Expo – Oral Solid Dose – USP <1062>.
- Natoli Scientific conducted a webinar on powder rheology and mechanics.

- Natoli Scientific conducted the Tablet & Capsule Process Development Training Class
- Natoli Scientific conducted the Tablet Development & Tooling Scale-Up Maintenance.
- Natoli Scientific conducted the Tablet Characterization with Regulatory Compliance Methods – USP<1062> Class.

- Tablet & Capsule
Process Development Training Class - Tablet Development & Tooling Scale-Up Maintenance
- Tablet Characterization with Regulatory Compliance Methods – USP<1062> Class

Undisputed Industry Expert in USP<1062> Tablet Characterization
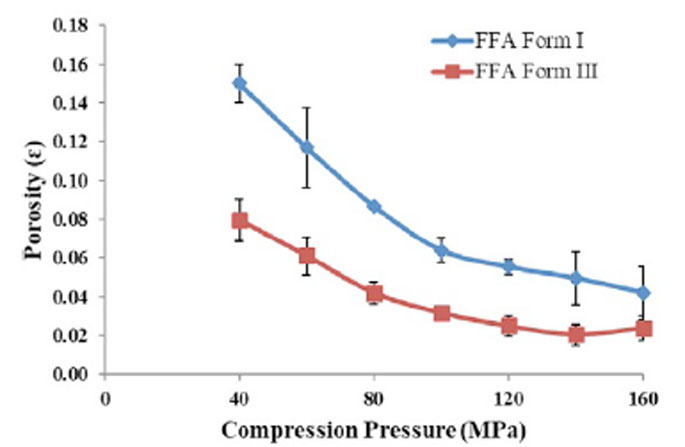
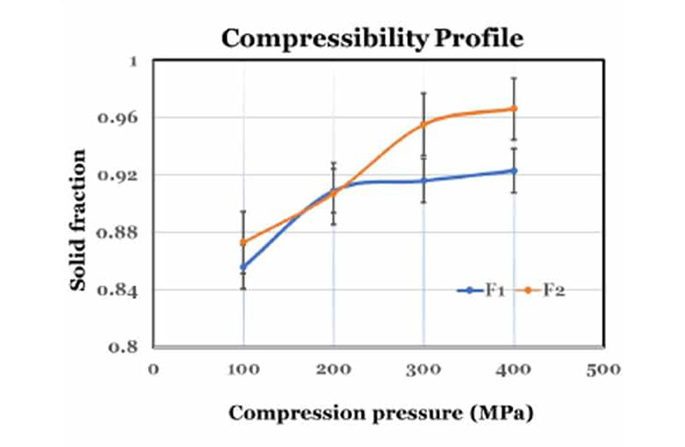
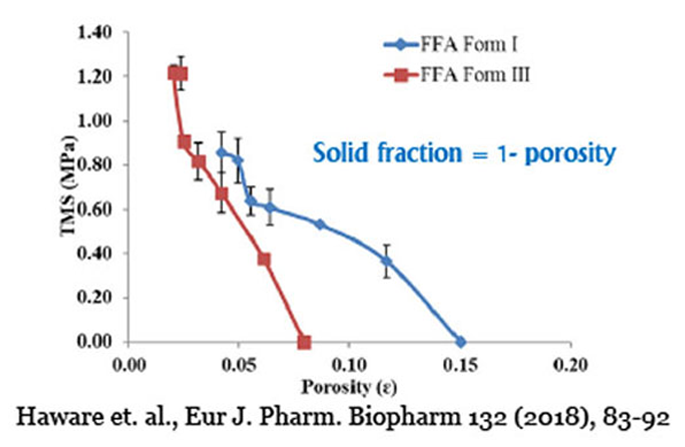
Compressibility: Solid Fraction and Tablet Porosity
Compressibility focuses on the relationship between solid fraction and applied compression pressure. Solid fraction quantifies the tablet's solid content relative to the powder's true density, while tablet porosity measures the air content within a fully compressed tablet. Through USP <1062> guidelines, you can optimize compression to achieve desired solid fractions and minimize tablet porosity, ensuring robust tablet mechanical strength.
API Polymorph Selection
Formulation Selection
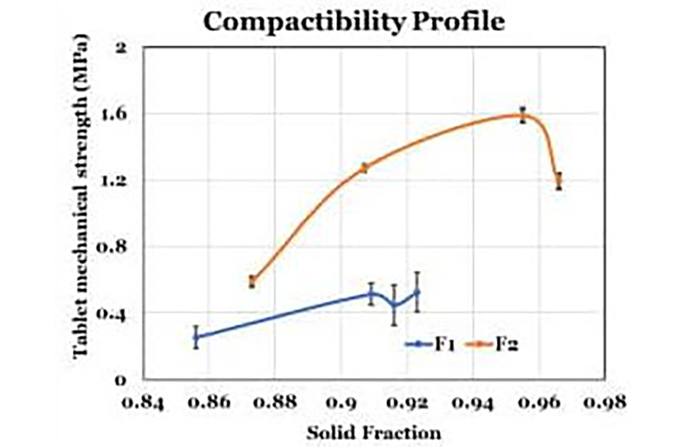
Compactibility: Tensile Strength And Solid Fraction
Tensile Strength and Solid Fraction Compactibility examines the relationship between tablet mechanical strength and solid fraction. Tablets with high solid fractions exhibit increased mechanical strength until critical limits are reached. USP <1062> provides guidance on optimizing compactibility to ensure tablets meet the desired strength requirements, while avoiding potential issues such as capping.
API Polymorph Selection
Formulation Selection
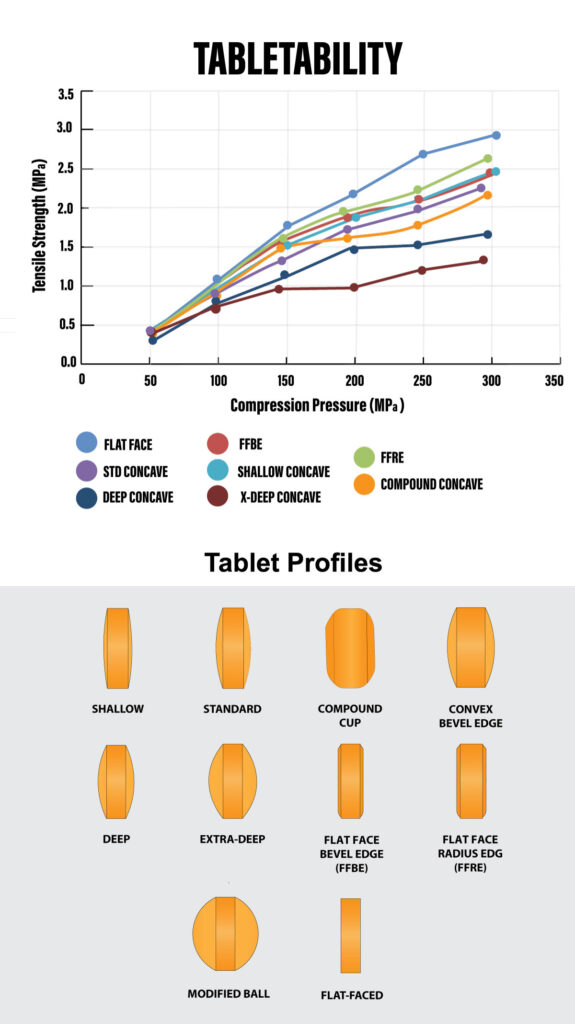
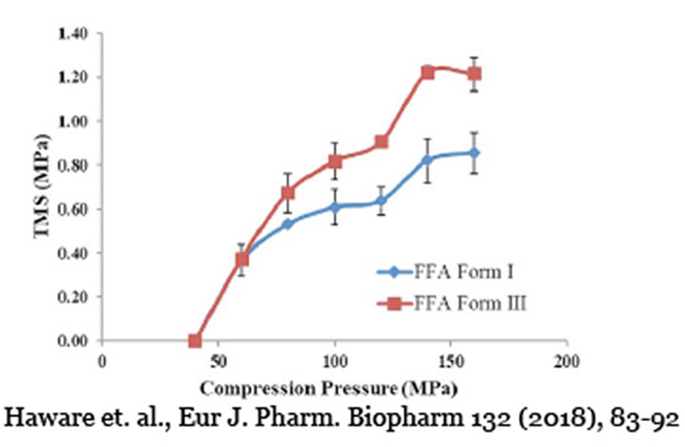
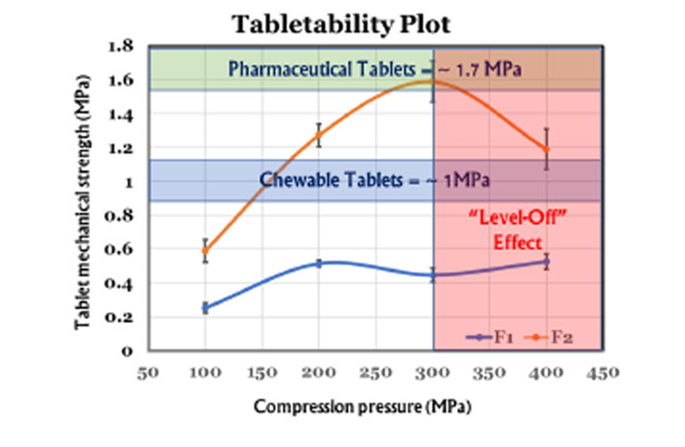
Tabletability: Mechanical Strength & Compression Pressure
Tabletability focuses on the relationship between tablet mechanical strength and compression pressure. Initially, as compression pressure increases, tablet mechanical strength improves. However, there reaches a point where it levels off. USP <1062> offers valuable insights to optimize compression pressure and achieve the desired mechanical strength while avoiding manufacturing challenges.
API Polymorph Selection
Formulation Selection
Learn More About USP <1062> and Why You Should Use It !

Tablet R&D Made Easy
The most cost effective, best value products in the industry.
The Natoli RD10A with AIM software is designed to use standard B and D tooling in either EU or TSM configurations, providing enhanced capabilities and up to 89kN of compression force. This state-of-the-art equipment allows you to streamline your tablet development process and unlock the full potential of your formulation.
Comprehensive Characterization: The Natoli RD10A enables you to gather precise data on tablet compression and performance using standard tooling. This comprehensive characterization allows for a deeper understanding of your powder’s behavior during compression, ensuring accurate and consistent tablet production.
Advanced Analysis: The AIM software complements the Natoli RD10A by providing advanced analytical capabilities. With AIM, you can analyze and interpret compression data, identify trends, and make data-driven decisions to optimize your tablet formulation.
Efficient Scale-up: When it comes to scale-up from R&D to production, the data collected with the Natoli RD10A and AIM software serves as a reliable foundation. You can confidently transition to larger-scale production, knowing that your formulation has been thoroughly characterized and optimized.
Enhanced Tablet Design: The use of standard tooling instead of flat-faced punches allows for a more accurate representation of your tablet’s final design during the formulation development stage. This consideration helps you avoid late-stage tableting issues and ensures that your tablets exhibit the desired quality attributes.
NP-400: The High-Speed Solution for Robust Tablet Production
In addition to the Natoli RD10A and AIM software, Natoli Scientific offers the NP-400, a high-speed rotary tablet press designed for efficient and robust tablet production. With its advanced technology and precise control over compression parameters, the NP-400 allows for reliable and scalable tablet manufacturing.
Revolutionize Your Powder Formulation with our Groundbreaking, Comprehensive Analysis.
For more information, call us at (215) 234-7376 / (636) 926-8900 or email rsedlock@natoli.com with POWDER ANALYSIS in the subject line.




