Raw Material Characterization
Inherent material deformation is a main player of any low API load or high API load tablet formulation. Formulations with a right blend of plastic-elastic-fragmenting material is necessary to form a robust viable compact.
Our instrumented presses can acquire real time compression force data under any compression condition if necessary. Our well-renowned material scientists are expert to transform this data into valuable information with several mathematical approaches including current USP <1062> chapter.
We will help you to understand an inherent material deformation of active pharmaceutical ingredient (API) and finding a right combination of excipients and a tablet manufacturing process for a successful transformation of API into a robust tablet.
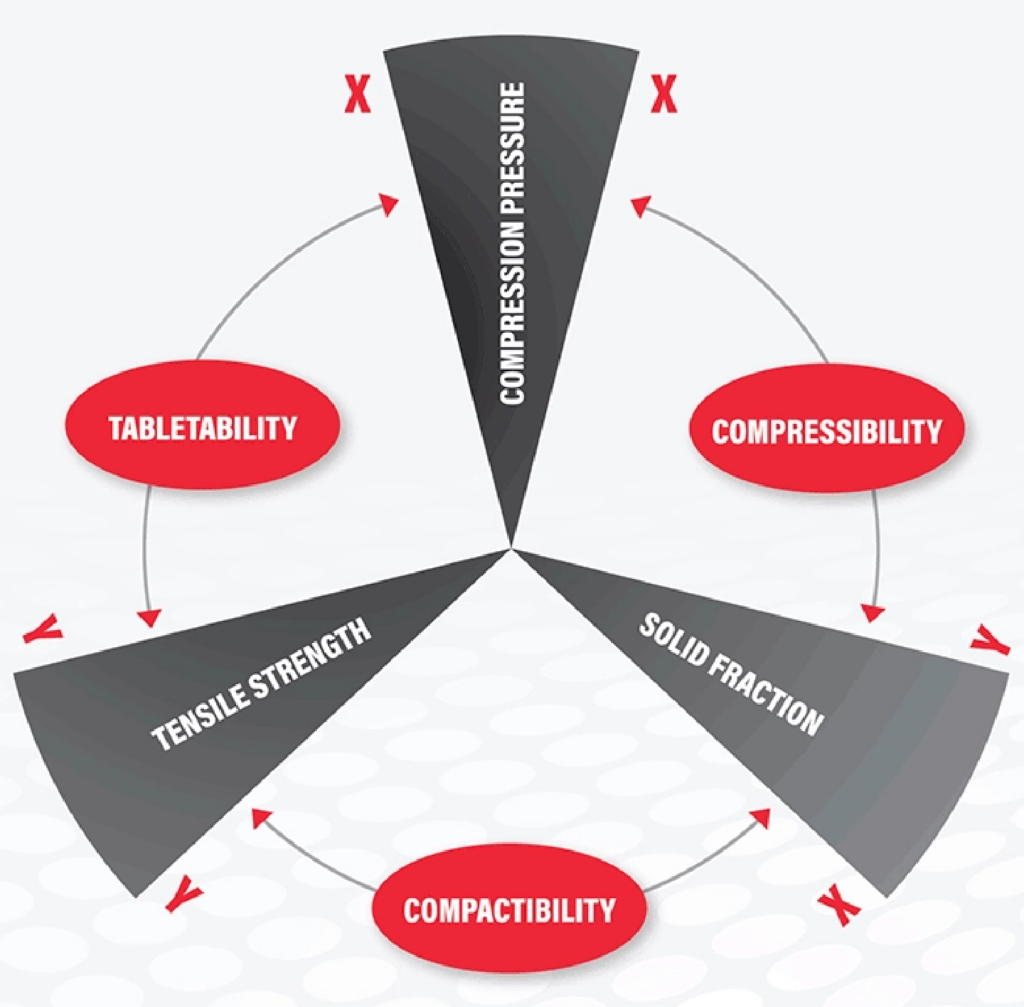
We understand that new lead molecules (API) are extremely expensive specifically in early development stage program. Therefore, getting maximum amount of valuable information with a minimum of API is extremely vital criteria for any testing in this phase. The USP<1062> testing will be done with a ‘material sparing approach’ using few grams of materials. We highly recommend doing individual API testing along with formulation blend if API amount in the final formulation is >30%. Bring us few grams of your API (If API load is about > 30%) and or formulation blend for testing. We will generate Compressibility, Compactibility, Tabletability and Manufacturing profile (USP<1062>) using different compression pressures as per the USP recommendation.

Our Natoli RD-10A is efficient semi-automatic machine to control experimental conditions for USP<1062> profiling. Formulation development scientist enjoys tremendous benefits after the inclusion of USP <1062> testing in early development program. It will predict possible success or failure of developed prototype lead formulations. This data will be your ‘guiding torch’ for the further formulation and process optimization, as well as subsequent development life cycle of your product.
Major Testing:
- USP <1062> testing (Compressibility, Compactibility, Tabletability and Manufacturability Profiling)
- Immediate Axial Recovery