Training in Action at Natoli Scientific
Training in Action at Natoli Scientific This week’s training sessions at Natoli Scientific brought together expertise, innovation, and hands-on learning. Highlights From the Sessions Powder Properties & Defect Prevention: Robert Sedlock emphasized the importance of characterizing raw materials to reduce common tablet defects like flow issues, segregation, capping, picking, and sticking. Tablet
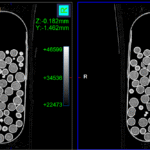
Advancing MUPS Technology with CT Scanning: A Non-Destructive First in Pharmaceutical Analysis
Advancing MUPS Technology with CT Scanning: A Non-Destructive First in Pharmaceutical Analysis Multi‑Unit Pellet Systems (MUPS) are an advanced class of multiparticulate oral solid dosage forms. Rather than delivering a single mass of active pharmaceutical ingredients, MUPS comprise numerous tiny, drug‑loaded pellets—each often coated for controlled release—and are then either filled into capsules or compressed
CT Scan Press Release
ST. CHARLES, MO., U.S. (June 23, 2025) – Natoli Engineering, a globally recognized innovator in the tablet compression industry, proudly introduces the Precision CT Scan. This innovative 2-D and 3-D imaging solution provides non-destructive analysis that allows manufacturers to visualize internal tablet structures without cutting or altering them, helping detect
Tablets and Capsules: Overcoming Similar Obstacles in Production
Challenges Faced by Tablets and Capsules: A Blog Post When it comes to the pharmaceutical industry, the development of solid dosage forms such as tablets and capsules involves intricate processes that are susceptible to various challenges. Both tablets and capsules share common hurdles that can significantly impact the production and
RESIDENCE TIME OF POWDERS IN TABLET COMPRESSION
Knowing how formulation moves through a tablet process is invaluable in optimizing the process for the greatest tablet quality and production. Residence time refers to the total time a particle or batch of material spends in a particular process or piece of equipment. In tablet compression, this refers
Unlocking Success – From Theory to Practice
In the world of tablet manufacturing, the phenomenon of “tablet sticking” has been a persistent challenge, often leading to production delays, quality issues, and increased costs. The science behind tablet sticking is complex, but understanding it is essential for pharmaceutical and nutraceutical companies seeking to optimize their production processes and
Unlocking Pharmaceutical Success with Natoli: From Idea to Reality
In the pharmaceutical industry, every capsule and tablet begins with a concept. Transforming that idea into a tangible product requires an expert manufacturing partner. Natoli stands out as a leader in pharmaceutical equipment and services, providing a comprehensive journey from formulation to full-scale production. Natoli Scientific: The Foundation of Formulation
The Role of Quality by Design in Pharmaceutical Tablet Development
In the dynamic realm of pharmaceuticals, Quality by Design (QbD) reigns as a transformative force. It’s more than just a concept; it’s the cornerstone of pharmaceutical excellence. Join us as we delve into the critical role that QbD plays in shaping the future of tablet development. Quality by Design (QbD)
It All Starts with an Idea…Unlocking Pharmaceutical Success with Natoli
Every capsule and tablet begins with an idea. It takes a professional and innovative manufacturing partner to turn that idea into a reality. Enter Natoli, a name synonymous with excellence in pharmaceutical equipment and services. From the early stages of formulation to the grand scale of full-blown production, Natoli offers
The Role of Quality by Design in Pharmaceutical Tablet Development
In the dynamic realm of pharmaceuticals, Quality by Design (QbD) reigns as a transformative force. It’s more than just a concept; it’s the cornerstone of pharmaceutical excellence. Join us as we delve into the critical role that QbD plays in shaping the future of tablet development. Quality by Design (QbD)
Onsite Preclinical Services to Support Your Product Development: Powder Micromeritics
Understanding the fine details of powder properties is crucial in the pharmaceutical industry, where the behavior of particulate matter can affect manufacturability and the efficacy of the final product. Natoli Scientific offers advanced on-site analysis of powder Micromeritics, which encompasses particle size distribution, flowability, and compaction properties. By assessing these
Exploring the Impact of Additives on Powder Tribocharging Behavior
Are you curious about how additives can influence the electrostatic charge build-up of excipients in pharmaceutical manufacturing? Join us on a journey to uncover the hidden dynamics of powder flow and electrostatic charge accumulation, a crucial aspect of powder processing that can significantly affect the quality and efficiency of pharmaceutical
Compaction Simulation / Emulation
Compaction Simulation / Emulation Building on the capabilities of the NP-RD10A benchtop tablet press (discussed in our previous article on USP <1062>), our services are further enhanced by the integration of the Presster™ compaction emulator. This state-of-the-art tool is designed to replicate the compaction dynamics of production tablet presses in
Batch & Lot Testing
Batch/Lot Testing for Release Batch and lot testing for release are critical steps in ensuring the quality and consistency of pharmaceutical tablets. Natoli Scientific provides comprehensive batch testing services that align with stringent industry standards and regulatory requirements. Each lot undergoes a rigorous analysis to verify that it meets predefined
