Formulation Testing Packages
Confidently Advance Your Formulation to Production
Avoid failed batches and costly delays. Get clear, data-driven insight before scaling up.
Natoli Scientific’s formulation testing packages help pharmaceutical and nutraceutical manufacturers reduce risk, accelerate development, and make informed decisions early in the process.
Standard Formulation Analysis Packages
Ideal for early stages, basic troubleshooting, or verifying formulation fundamentals.
Tablet Formulation Snapshot
$475
Designed for: Early-stage formulation development and identifying potential risks before pilot-scale work.
You’ll learn:
- Powder Flow Testing: Bulk Density, Tapped Density, Hausner Ratio, Compressibility Index
- Powder Manufacturability: USP <1062> Manufacturability Plot
- Moisture Content (LOD)
Outcome:
A clear snapshot of whether your formulation meets baseline expectations for compression and stability.
Tablet Compression Analysis
$1,475
Designed for: Teams evaluating a powder’s performance during compression to predict scale-up behavior and manufacturing reliability.
You’ll learn:
- Basic Powder Micrometrics: Bulk Density, Tapped Density, Hausner Ratio, Compressibility Index
- USP <1062>: Compressibility, Compactibility, Tabletability, Manufacturability
- Particle Size Distribution
- Helium Density
- Moisture Content (LOD)
Outcome:
Data-driven understanding of how your formulation will run on a press and what adjustments may be required.
Encapsulation Analysis
$875
Designed for: Powders used in capsule production. Optimizing flow, fill weight, and uniformity.
You’ll learn:
- Powder Micrometrics: Bulk Density, Tapped Density, Hausner Ratio, Slug Tester
- Manufacturability: Optimize Capsule Size and Weight, Asses Potential for Product Loss, Identify Unnecessary Fillers, Maximize Drug Load.
- Particle Size Distribution
- Moisture Content (LOD)
Outcome:
Verification that your formulation is suitable for capsule filling or identification of what must change.
Why Your Formulation Needs Early Testing
When formulations fail, production stops—costing time, money, and momentum. Weak compression, sticking, poor flow, and inconsistent strength are common, predictable issues that derail development.
You need clear data and expert guidance before scaling. Natoli Scientific provides formulation testing backed by decades of tooling, process, and mechanical analysis experience. Our team helps you understand exactly how your material will behave—and what it needs to succeed.
How It Works
- You Send Us Your Material
- We Test, Analyze, and Report
- You Receive Clear, Actionable Guidance
Advanced Formulation Analysis Packages
Ideal for complex formulations, products entering scale-up, or investigations requiring deeper mechanical modeling.
Pilot
Package
$7,493
Best for: Predicting how your formulation will scale from R&D to pilot or full production.
Includes:
- USP <1062> Tablet Compression Characterization & Powder Micromeritics
- Strain Rate / Speed Study Characterization (1 Shape)
- CT Sans (2 Samples)
- TaBlitz Software License (12 months)
Outcome:
A complete roadmap for successful scale-up, including optimal press settings and risk mitigation strategies.
Scientific
Package
$19,490
Best for: High-risk development, challenging APIs, or situations requiring deep investigation and expert-level interpretation.
Includes:
- USP <1062> Tablet Compression Characterization & Powder Micromeritics (5 shapes)
- Strain Rate / Speed Study Characterization (2 Shapes)
- Direct Compaction Simulation: Heckel Plot, Force Displacement Work Curves, Deformation Property Analysis
- CT Sans (5 Samples)
- TaBlitz Software License (12 months)
Outcome:
A definitive understanding of how your formulation behaves, why, and what exact changes will ensure success in production.
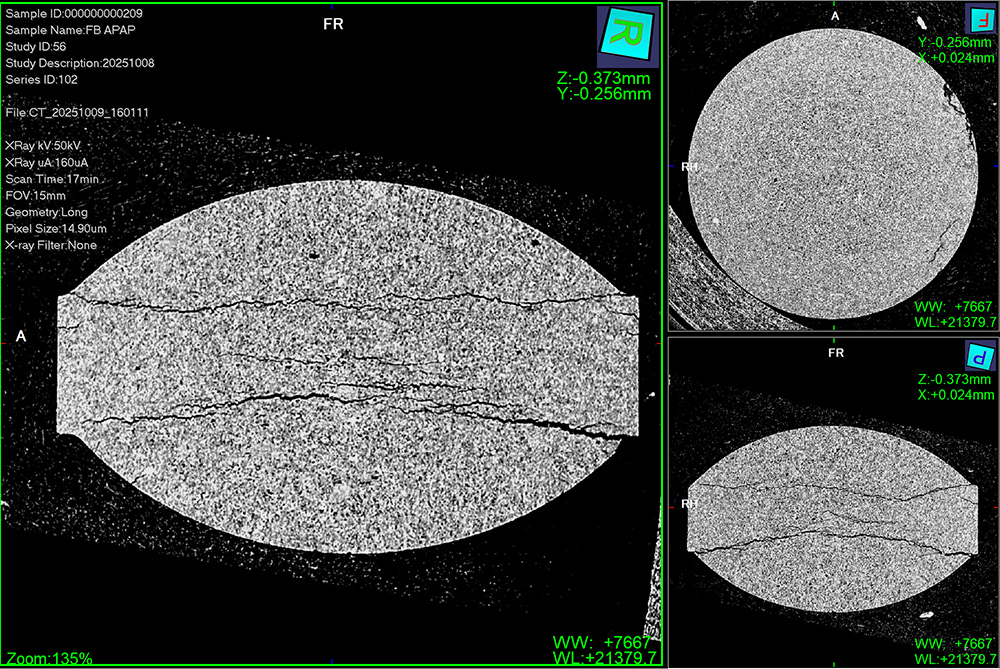
CT Scanning for Advanced Structural Insight
CT scanning delivers non-destructive, high-resolution imaging of tablets or capsules. It’s ideal for identifying internal defects, density variations, voids, coating issues, or core-shell inconsistencies.
CT Scanning is recommended when:
- Tablets fail unexpectedly
- You need structural insight beyond mechanical data
- You’re testing coated, layered, or complex products
- You want a visual confirmation of internal uniformity
Need Something More Specialized?
Natoli Scientific can customize any testing package to fit your formulation and production needs. Our laboratory provides extensive analysis for:
- Root cause analysis of compression and encapsulation failures
- Powder flow and lubrication sensitivity studies
- Strain rate and material deformation evaluations
- Scale-up and process optimization
Tailored testing to ensure your oral solid dose is production-ready.
Our custom formulation testing services focus on meeting your unique requirements. Comprehensive laboratory services are provided to improve processes, decrease development times, and avoid scale-up challenges and manufacturing issues.
Our approach ensures your formulation meets the necessary validation requirements, helping you achieve reliable, high-quality results tailored to your specific production needs.
Move Forward With Confidence
Don’t risk delays, failed batches, or costly reformulations. Get clear data, expert analysis, and actionable guidance that helps you bring a successful product to market.
