Advancing MUPS Technology with CT Scanning: A Non-Destructive First in Pharmaceutical Analysis
Multi‑Unit Pellet Systems (MUPS) are an advanced class of multiparticulate oral solid dosage forms. Rather than delivering a single mass of active pharmaceutical ingredients, MUPS comprise numerous tiny, drug‑loaded pellets—each often coated for controlled release—and are then either filled into capsules or compressed into tablets.
Why MUPS Matter
- Enhanced drug delivery control: Pellets can be engineered with coatings that provide immediate, delayed, or extended release, and can be mixed within one dose to achieve biphasic or multiphasic release profiles.
- Improved physiological distribution: The dispersion of many small pellets reduces variability in transit and absorption, lowers the risk of localized irritation, and mitigates the dangers of dose dumping.
- Patient-centric design: MUPS are particularly useful for sprinkle formulations, helping those with swallowing difficulties—such as pediatric or geriatric populations—by facilitating administration via soft food or beverages.
- Formulation flexibility: Drug layering onto inert starter cores like sugar spheres, microcrystalline cellulose (MCC), isomalt, or newer materials like calcium phosphate allow for customizable design depending on therapeutic and mechanical requirements.
Manufacturing Challenges
Producing MUPS presents a critical challenge: during compaction into tablets, the pellet coatings can crack or become damaged, which may disrupt controlled-release functionality. To address this, formulators experiment with pellet core materials and compaction pressures. For example:
- MCC-based pellets generally withstand compaction better than those made with crospovidone, which can deform more and lead to greater coating damage.
- Data‑driven modeling approaches such as DRSA (Dominance‑Based Rough Set Approach) have been used to optimize formulation and compression settings to preserve release profiles.
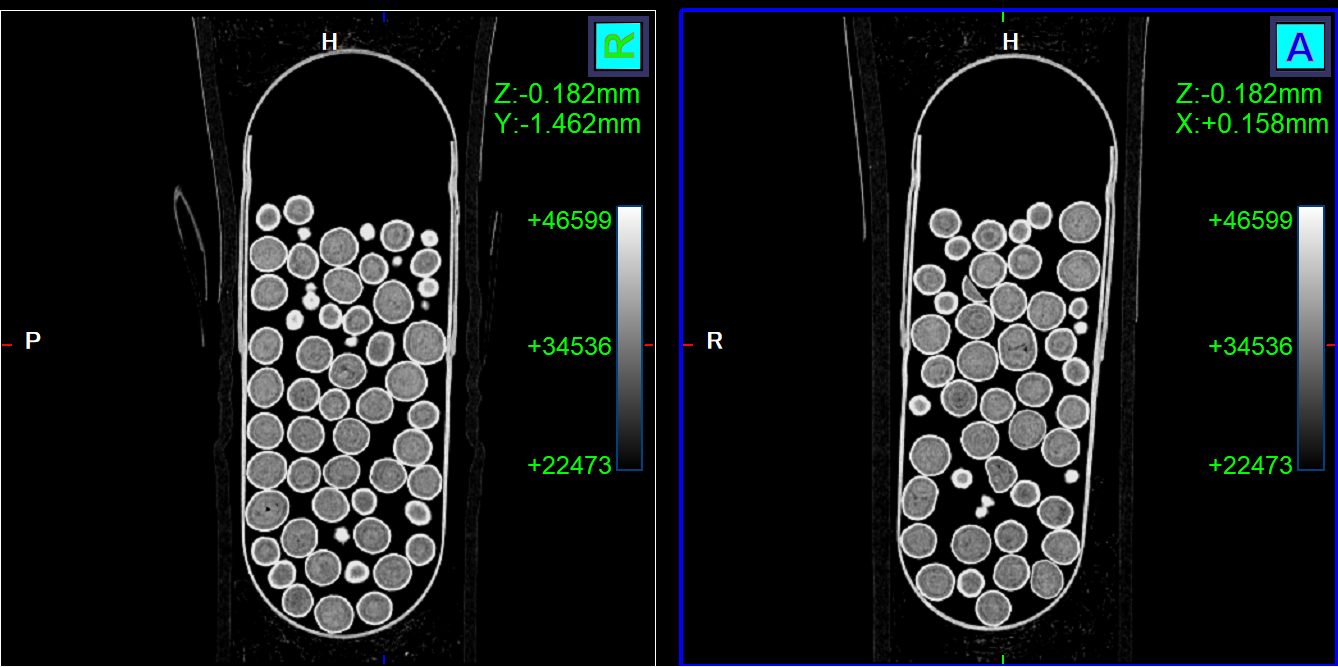
Why CT Scanning Changes the Game
Until now, the evaluation of pellet integrity and distribution in MUPS required destructive analysis—often slicing or dissolving the formulation. Natoli Scientific is pioneering the first-ever application of CT scanning for non-destructive analysis of MUPS, enabling:
- High-resolution, 3D visualization of pellet dispersion in both tablets and capsules—without destroying the dosage form.
- Internal defect detection and quantification of cracks, voids, or pellet clustering.
- Non-destructive, data-rich assessment, preserving samples for further analysis.
- Improved quality control and formulation confidence, especially for scale-up and regulatory validation.
Putting It All Together: Educational Depth Meets Innovative Capability
MUPS represent a sophisticated deliverable in modern pharmaceutical design—offering controlled release, flexibility, and patient-friendly dosing. Yet, their structural complexity demands advanced evaluation tools. With CT imaging, Natoli Scientific bridges that gap, offering a scientifically rigorous, non-invasive technique to validate MUPS integrity and performance.
